Based on the years IoT industry niche of OEM in Taiwan, Hukui Biotechnology Co., Ltd. integrates the medical professional knowledge of biotech nowadays, not only can take electric ODM/OEM contract, but also produce contracted medical device. At the same time, Hukui team has solid regulation ability with sound quality management system, and we can communicate with customers each other in different country by using standard documents. As the result, we increase working efficiency and reduce mistakes from product design and development procedure to QC/QA process during the production.
About Us
Based on the years IoT industry niche of OEM in Taiwan, Hukui Biotechnology Co., Ltd. integrates the medical professional knowledge of biotech nowadays, not only can take electric ODM/OEM contract, but also produce contracted medical device. At the same time, Hukui team has solid regulation ability with sound quality management system, and we can communicate with customers each other in different country by using standard documents. As the result, we increase working efficiency and reduce mistakes from product design and development procedure to QC/QA process during the production.

Product
The two core products, Handheld Ultrasound(POCUS) and Medical Endoscope, Hukui takes the advantages of outstanding engineering team, excellent production team, and rigorous regulation team in Taiwan, rapidly applies ODM/OEM ability to medical device development procedure. This strength will definitely not only reduce the development cost and design risk, but also increase the successful opportunity and time efficient.
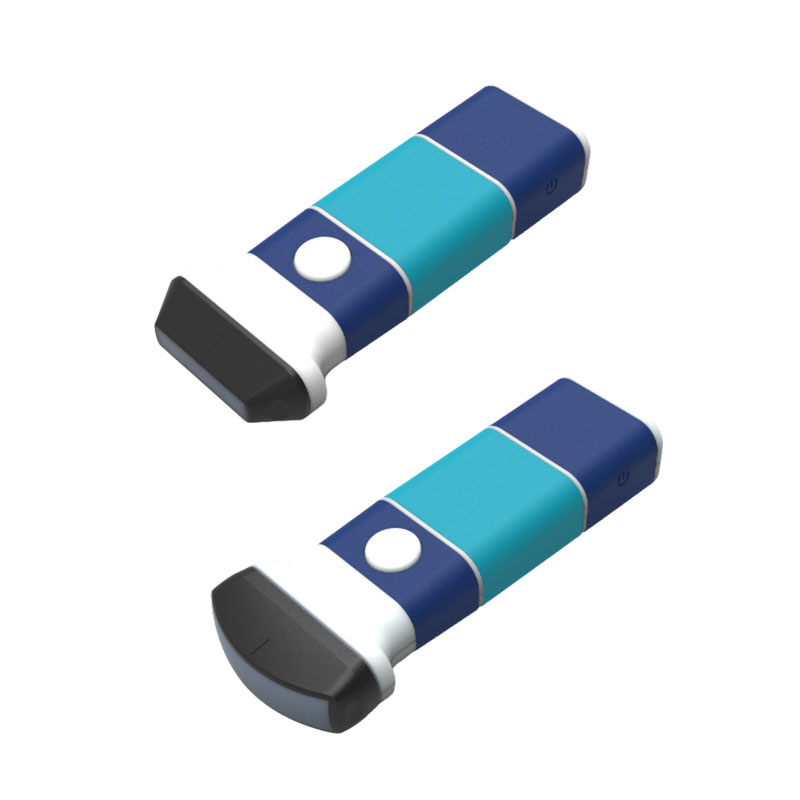
Handheld Ultrasound
Medical device with multi-country license is a feasible registration strategy in the industry. It can take advantage not only in the international trading business but also in the capital market to raise fund.
Endoscope
Hukuibio has extensive experience related to medical electronic design and devices. We have successfully designed and built a number of medical related projects including; an optical image device , an ultrasound device and a handheld telemedicine device.
Service
We focus solely on client needs, providing engineering sample, finished product and status updates throughout the lifecycle of the project. The lifecycle is from the transfer in the existing spec for GMP mass production, to a full project of POC, EVT, DVT and PVT process.
CDMO(ODM/OEM)
Consultant
Registration
Sales
Press
Welcome to find more Hukui news on the social media.