Medical device with multi-country license is a feasible registration strategy in the industry. It can take advantage not only in the international trading business but also in the capital market to raise fund. A professional RA team can lead the medical device company for saving learning time and maximizing the resource. For any device, no matter it is well-known for normal use or with innovation and unique intended use, to prepare registering in advance will help explore the market of product and extend the potential business channels.
EU Registration
US Registration
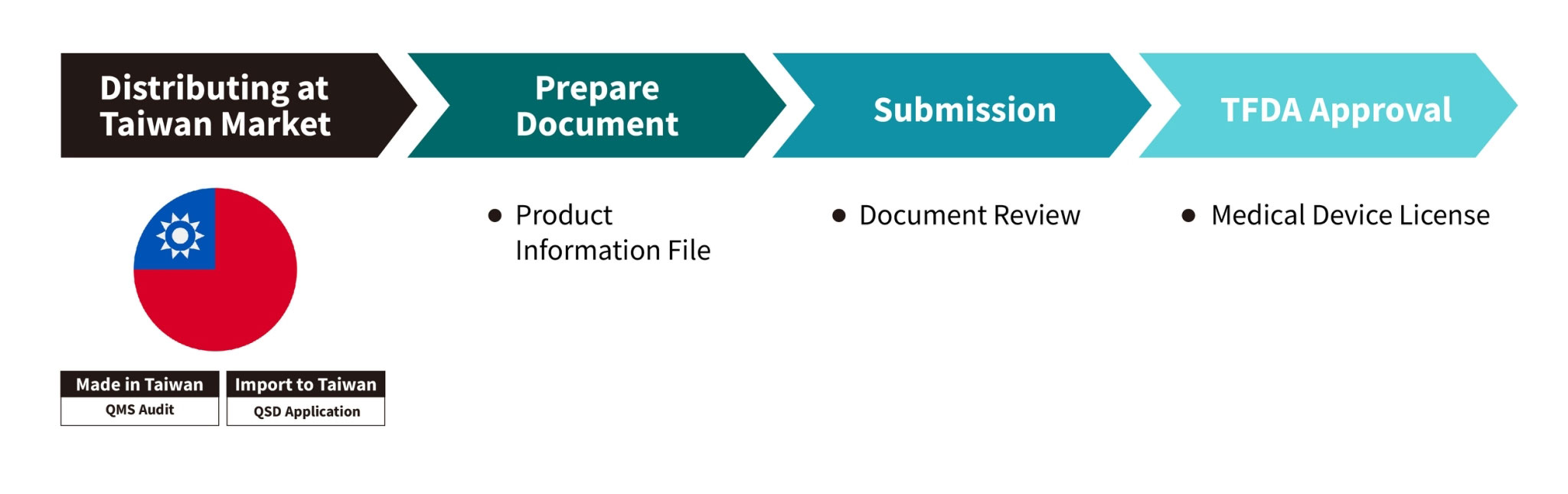
Taiwan Registration
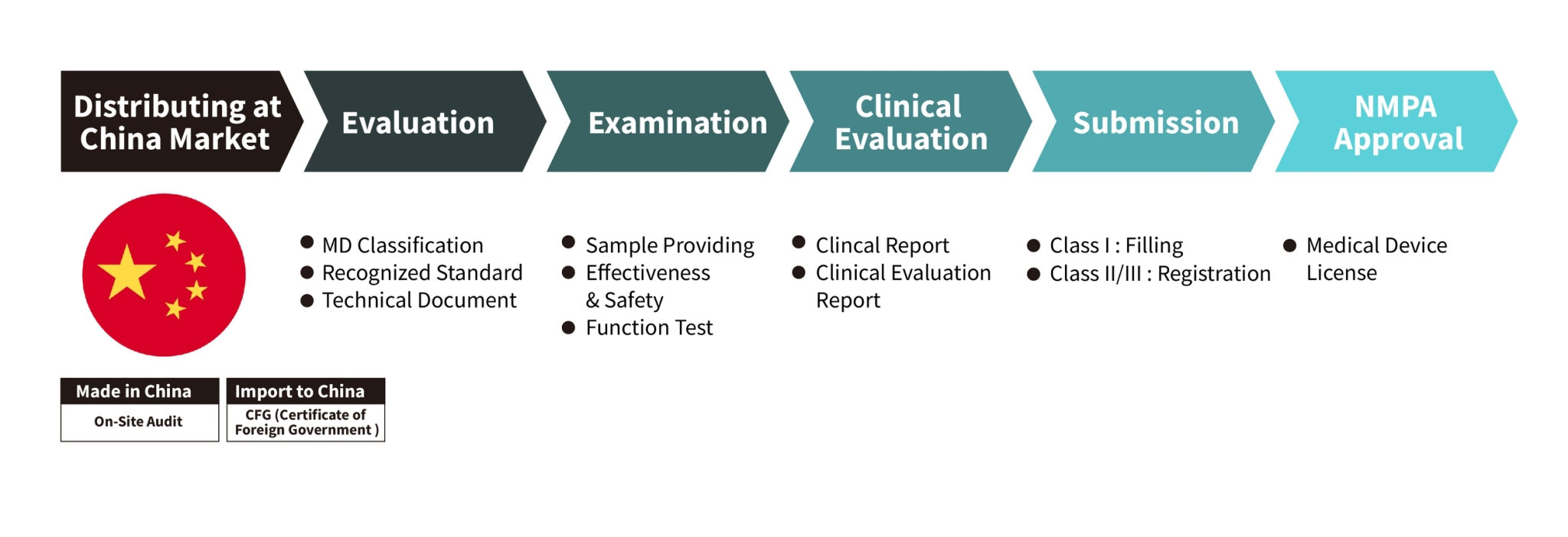
China Registration