Class Ⅰ :includes medical devices with the lowest risk, where safety and effectiveness can be ensured through routine administration. Only filing is required.
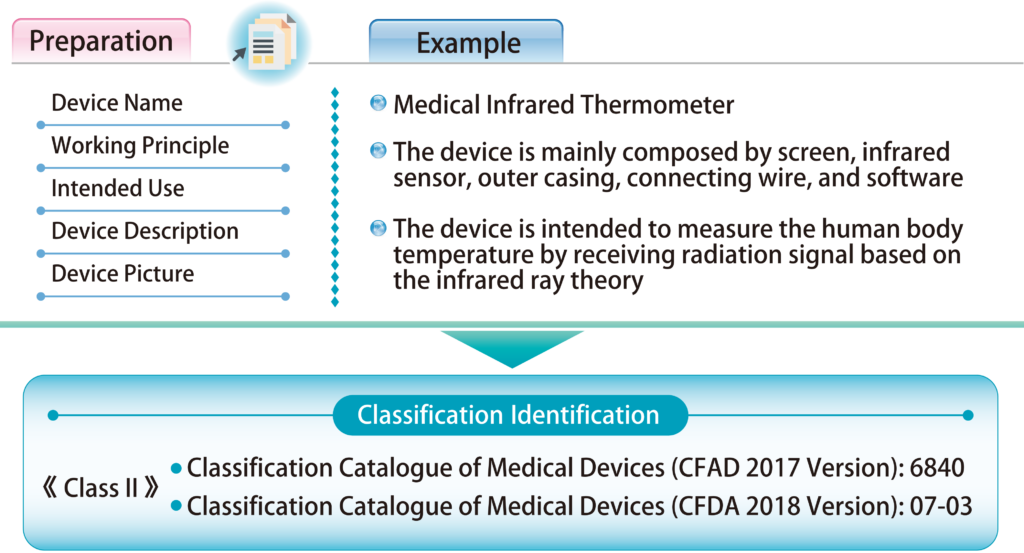
Class Ⅱ :includes medical devices with moderate risk, where strict control and administration are required to ensure their safety and effectiveness. Registration is required.
Class Ⅲ: includes medical devices with the highest risk and must be strictly controlled and administered through special measures in respect to safety and effectiveness. Registration is required.
Innovative, Prior, and Drug-Device Combination Products:Those belonging to innovative, prior, and drug-device combination products, when entering corresponding process, can be immediately assigned to product classification.
HUKUIBIO invites you to contact us at [email protected] to discuss your medical device development efforts in China, including regulatory impacts that may impact your intended commercialization strategies.

